Antiseptics and disinfectants
Antiseptics and disinfectants are antimicrobial agents. Antimicrobials also include chemotherapeutic substances used to treat infectious diseases, which are discussed in a special section (see below).
Studies to determine the possible relationship between antibiotic resistance and resistance to disinfection of clinical isolates are rare. The exact role of plasmids in disinfection resistance and the possibility of choosing disinfectants for antibiotic resistance is unknown.
Nosocomial infections have become a major cause of morbidity and mortality worldwide. Adequate hygiene practices and environmental disinfection are essential to control these infections. The aim of this study was to evaluate the bactericidal activity of some disinfectants in resistant and antibiotic-susceptible hospital samples. Susceptibility to disinfectants and antibiotics from 27 clinical specimens was determined by the use dilution method and the Kirby-Bauer method, respectively.
The term " antiseptic" comes from two Greek words: anti - against, sepsis - putrefaction. The founder of the doctrine of antiseptics is Lister, who, having come to the conclusion that the cause of wound infections is the contamination of wounds by microorganisms inhabiting the air, began to use (1867) local carbolic acid for the treatment of wounds. The term "disinfection" was proposed by R. Koch. By disinfection, Koch understood the destruction of pathogens in the environment using chemical and physical methods of exposure.
The results showed that all test samples were susceptible to sodium hypochlorite, glutaraldehyde and quaternary ammonium-formaldehyde-ethyl alcohol association. However, the susceptibility of the samples to phenol and quaternary ammonium turned out to be variable. Among 21 antibiotic resistant samples, eleven and eight were resistant to quaternary ammonium and phenols, respectively. Among the six samples that showed susceptibility to antibiotics, two samples showed resistance to these disinfectants.
Currently, antiseptic agents are understood to mean substances used for local effects mainly on pyogenic flora in the treatment of festering wounds, boils, carbuncles and other diseases. As a rule, these substances are not used for the treatment of common infections, since many of them, being general cellular poisons, being absorbed into the blood stream, have a toxic effect on the body. In addition, antiseptics are used as preservatives in the food industry, as well as in the manufacture of dosage forms.
The results obtained in this study indicated that there was no correlation between antibiotic susceptibility and disinfectant susceptibility in the analysis of hospital clinical specimens. Key words: disinfectant activity, antibiotic activity, hospital samples.
Susceptibility of vancomycin-resistant enterococci to environmental disinfectants. Association of Official Analytical Chemists. Problems of disinfection in hospitals. Centers for Disease Control and Prevention. Disinfectant tolerance and antibiotic resistance in psychotropic Gram-negative bacteria isolated from vegetables. Comparative susceptibility of hospital isolates of gram-negative bacteria to antiseptics and disinfectants.
The division of the agents under consideration into antiseptic and disinfectant is largely arbitrary, since the same substances can be assigned to both groups.
Antiseptic substances, depending on a number of conditions (see below), have both bacteriostatic and bactericidal effects on microbes. The difference between them lies in the degree of the effect. Bacteriostatic action is said to be in the case when, under the influence of an antiseptic, the reproduction of microorganisms is temporarily delayed, although their viability is preserved. If, when exposed to a substance for short term the death of most microbes occurs, then this effect is called bactericidal.
Resistance to heavy metals and disinfectants in clinical Gram-negative rod isolates. Resistance to multidrug resistance to antiseptics and disinfectants in coagulase-negative staphylococci. Antiseptics and disinfectants: activity, action and resistance.
Effect of chlorination on antibiotic resistance profiles of wastewater-associated bacteria. Application. National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disc susceptibility tests.
Power of action antiseptics, as already mentioned, depends on many conditions. First of all, different microorganisms have different sensitivity to different drugs of this group. The concentration of the substance is of great importance: at low concentrations of the drug, a bacteriostatic effect occurs, with an increase in concentration, a bactericidal effect often develops, and the rate of death of microbes increases with increasing concentration of the substance. The temperature factor has a great influence on the manifestation of antimicrobial action. As the temperature rises, the process of microbial death accelerates. Antimicrobial action largely depends on the duration of exposure to the drug: the longer the time of action, the more pronounced the effect. The presence of protein in the medium reduces the bactericidal effect of the drug. The degree of effectiveness of an antiseptic also depends on its solubility in water and lipids, on the distribution coefficient between lipids and water. A number of dependencies between the chemical structure and the action of a substance have been established.
Minimum inhibitory and bactericidal concentrations of some antiseptics and disinfectants against strains of hospital origin. Review of nosocomial infection control in Brazil. Bacterial resistance to antiseptics and disinfectants.
Plasmids and bacterial resistance to biocides: a review. Disinfection, sterilization and waste disposal. Susceptibility of antibacterial and antibiotic-resistant bacteria to disinfectants. Infection. Antiseptic and antibiotic resistance in Gram-negative bacteria causing urinary tract infection.
According to modern concepts, antimicrobial agents have a different mechanism of action on microorganisms, which largely depends on both the chemical and physicochemical properties of the drugs. The strength of the antimicrobial action of acids, alkalis and salts is largely due to their ability to dissociate. Other things being equal, a substance that dissociates to a greater extent will be more active against microbes than a substance with a low degree of dissociation. The antimicrobial effect of other substances is based on the fact that they lower the surface tension of the medium. Recently, great importance in the mechanism of action antimicrobial agents give them the ability to block sulfhydryl groups (-SH) of microbes, as well as to join with the active groups of enzymes. Under the influence of antiseptics, the process of cell division is disrupted, and therefore morphological changes are observed, manifested by a change in the shape of microbes, a violation of the structure of the cell itself. Morphological changes caused by various antiseptics are heterogeneous. Antiseptic and disinfectants inhibit the activity of many bacterial enzymes. In particular, it has been established that there is a close parallelism between the bactericidal action of substances and their ability to inhibit the dehydrase activity of bacteria.
Transposition of gentamicin resistance to staphylococcal plasmids encoding resistance to cationic agents. Postal address: Institute of Microbiology Prof. An antiseptic is chemical substances, which are used to kill a pathogenic microorganism in or on surface tissue. Antiseptics mainly work by dissolving cell membranes, denaturing protein, and causing cell dehydration due to evaporation. For example, chloroxylenol is a chlorinated phenolic antiseptic primarily active against Gram-positive bacteria and is used in vaginal examination lubricating creams; used on obstetrical forceps, etc.
A very large number of different substances are used as antiseptics and disinfectants. Intensive work is underway to create new drugs. In this regard, there is a need to compare drugs with each other in terms of activity. For this purpose, minimum bacteriostatic and bactericidal concentrations of drugs are established. Antimicrobial activity is usually expressed by the phenol coefficient. To determine it, the concentration of phenol, which has a bactericidal effect, and the concentration of the test substance solution, which causes the same effect, are established. The ratio of the concentration of phenol to the concentration of the test substance is called the phenol coefficient.
Disinfectants are chemicals that are used to kill a pathogen on or on the surface of objects. Disinfectants primarily work by destroying cell membranes and denaturing cell proteins and enzymes. These chemicals are very effective against vegetative Gram-positive and Gram-negative bacteria, mycobacteria and viruses. For example, lysol phenolic compound is used as a general disinfectant for home or hospital use such as disinfection of floors, bathrooms, wash basins, organic waste such as phlegm, feces, urine, etc.
Of great importance for the evaluation of an antiseptic is also its general toxicity to the animal organism. For medical practice, drugs with the least toxicity are of the greatest value.
Antiseptics and disinfectants are very diverse in nature, so their classification is rather difficult. For convenience of presentation, we have adopted the division of preparations according to chemical characteristics. In some cases, substances are combined into groups based on other characteristics.
The most commonly used products in clinical practice today include povidone iodine, chlorhexidine, alcohol, acetate, hydrogen peroxide, boric acid, silver nitrate, silver sulfadiazine, and sodium hypochlorite. The global antiseptics and disinfectants market is expected to experience high growth during the forecast period due to the increasing share of hospitals and advanced healthcare facilities around the world. In addition, rising health club and fitness center trends are expected to drive the market for antiseptics and disinfectants.
Halides
Chlorine
Chlorine and some of its compounds have a strong bactericidal effect. A chlorine concentration of 0.02 mg/l is sufficient to kill a wide variety of microbes. In an environment rich in organic substances, the bactericidal effect of chlorine is reduced, since in this case part of it is bound by substances in the environment, and the active concentration of chlorine decreases.
In addition, the great interest of the younger generation in taking precautions against influenza, bacterial and viral infections is likely to increase the demand for antiseptics and disinfectants. However, tight regulation and a saturated market in developed countries may act as a constraint on the growth of the antiseptic and disinfectant market.
On the basis of type, the global market for antiseptics and disinfectants is divided into six types: alcohols and aldehydes, phenols and derivatives, bigunaides and amides, quaternary ammonium compounds, iodine compounds, and others. The growing vibrant interest in healthcare is another major factor that is expected to drive the antiseptic and disinfectant market.
The mechanism of the bactericidal action of chlorine is due, on the one hand, to the fact that it enters into combination with the proteins of the protoplasm of microorganisms, forming substances such as chloramines, from which free chlorine is easily split off:
R-CO-NH-R1+Cl2 --- RCONClR1+HCl.
On the other hand, when chlorine is dissolved in water, it reacts with it, and in the end, oxygen is released, which at the time of release has strong oxidizing properties:
In the end user segment, the institutional segment dominates the global antiseptic and disinfectant market. Globally, about 50% of the antiseptic and disinfectant market was recorded by the institutional end-user segment. This growth is mainly due to the presence of a large number of hospitals, combined with an increase in the number of medical specialists around the world. The hospitals and healthcare sector are expected to experience significant growth during the forecast period due to the outbreak of infectious diseases such as swine flu and bird flu, which has triggered the use of the market for antiseptics and disinfectants in developing countries.
Cl2+H2O = HCl+HClO
HClO = HCl+O
Thus, the action of chlorine is based either on chlorination or on the oxidation of organic substances.
Either free chlorine or substances containing the so-called active chlorine, i.e. chlorine, which is easily split off in the atomic state, has the described action. Chlorine ions, as well as chlorine atoms, firmly bound in organic or inorganic compounds, do not have this effect.
It is also expected that the domestic use of antiseptics and disinfectants will have a significant increase due to increased public awareness of the potential dangers of microbial infections. North America is expected to experience moderate growth over the forecast period due to saturation of the red meat market in the US and Mexico with antiseptic and disinfectant. This is expected to have a negative impact on the antiseptic and disinfectant market over the years.
However, the demand for antiseptic and disinfectant has been growing in the past few years due to the growing awareness of the nutritional benefits of antiseptic and disinfectant. This is expected to promote the growth of the antiseptic and disinfectant market. Besides, high level literacy combined with an increase in the number of health centers and a business center is likely to contribute to the growth of the antiseptic and disinfectant market.
Of the chlorine-releasing compounds, bleach, consisting of Ca(ClO)2, CaC12 and Ca(OH)2, is used for external disinfection, as well as a deodorizing (odor-destroying) agent. Bleach causes discoloration of fabrics, so it should not be used to disinfect clothing. Chloric lime is unsuitable for processing metal objects, as it causes metal corrosion.
Europe has been the leading region for the global market for antiseptics and disinfectants. In the last decade, this region has seen a marked increase in the use of antiseptics and disinfectants due to increased awareness of pandemic diseases such as the spread of influenza and viral infections.
Asia Pacific is expected to be the fastest regional segment for the market natural antiseptics during the forecast period due to the widespread use of Ayurveda in this region. The growing number of the medical sector, combined with the literacy rate, mainly in China, India, Indonesia and Thailand, drives the antiseptic and disinfectant market in this region. In addition, increased awareness of personal care and hygiene maintenance in emerging economies China and India is expected to increase the growth of the antiseptic and disinfectant market in this region in the near future.
For hand disinfection, only relatively weak solutions (not more than 1-2%) can be used, since bleach irritates tissues. A more convenient form of using bleach for disinfecting skin and wounds is the Carrel-Dekin liquid, made according to a special recipe: 20 g of bleach and 14 g of soda are shaken in 1 liter of water; after settling, the liquid is filtered and the filtrate is neutralized with 4 g of boric acid. In surgical practice, for the treatment of wounds, preference is given to drugs that slowly release chlorine, due to which their irritating properties are reduced. These include chloramine B - sodium benzenesulfochloramide. Pantocid (paradichlorosulfamidobenzoic acid) is used mainly for water disinfection, as well as for disinfecting hands, douching and treating wounds. Pantocide is also used in contraceptive preparations.
In this report, the Global Antiseptics and Disinfectants Market Segment is presented as follows. Global Market for Antiseptics and Disinfectants: Type Analysis.
- Alcohol and aldehydesPhenols and derivatives Biguanides and amides.
- Quaternary ammonium salts.
- iodine compounds.
- Other.
Internal end users Institutional end users. . The market for global antiseptics and disinfectants: a regional analysis. Telemedicine market: medical education market: therapeutic vaccines market: teleradiology market: biomaterials market. We create futuristic, cutting edge, informative reports ranging from industry reports, company reports to country reports. We provide our clients with not only market statistics published by public private publishers and public organizations, but also trendy and latest industry reports, as well as outstanding and niche company profiles.
Iodine
The antimicrobial action is inherent in free iodine, but not in iodides. The phenol coefficient of iodine is 180-230. Iodine is detrimental to many types of microorganisms. Noteworthy is the fact that pathogenic fungi are sensitive to the effects of iodine. The bactericidal effect of iodine is due to both the suppression of the enzyme systems of microbial cells and the denaturation of proteins and is associated with its iodinating and oxidizing effects.
Our database of marketing research reports includes a wide range of reports from cardinal industries. Our database is constantly updated so that our customers can quickly and directly access our database. Keeping in mind the needs of the client, we have included expert information on global industries, products and market trends in this database.
Classification of antiseptics and disinfectants for humans
Last but not least, we are committed to ensuring the success of the clients associated with us - after all, if you succeed, a little light shines on us.
Thomas Flettoto
The classification of these products is an individual decision requiring consideration of all characteristics of a particular product. The classification depends in particular on factors such as claims, presentation of the product, composition, mode of action and mode of application.Iodine is widely used in surgical practice for primary processing wounds, surgical field and surgeon's hands, as well as an antifungal agent in the treatment of skin diseases caused by pathogenic fungi.
Locally on the tissue, iodine has an irritating effect. In some individuals, idiosyncrasy to iodine is observed, which is expressed by the appearance of a rash, and fever.
Inside, iodine is prescribed for the prevention of atherosclerosis, in the treatment of syphilis, and in small quantities in hyperthyroidism (see Drugs that affect metabolism).
Of the iodine compounds used as antiseptics, one should point to iodoform (triiodomethane). In contact with living tissues, free iodine is released from iodoform, which has antiseptic action, iodoform used to be very widely used to treat infected wounds and ulcers. Nowadays, it is used relatively rarely due to its very strong smell.
Preparations
Chlorine lime (Calcium hypochlorosum), FVIII. White powder with a specific smell of chlorine. The content of active chlorine must be at least 25%. Chlorine-lime milk is prepared from bleach (1-2 parts of bleach to 8-9 parts of water), which is used to make working solutions of the required concentrations.
Chloramine B (Chloramimim B), FVIII. White crystalline powder with chlorine odour. Contains 25-29% active chlorine. In surgical practice, 1-2% solutions are used for wound treatment, 0.25-0.5% solutions are used for hand disinfection, 2-5% aqueous solutions are used for skin dehydration and external disinfection.
Pantocide (Pantocidum), FVIII. White powder with a slight smell of chlorine. Contains at least 48% active chlorine. It is produced in the form of tablets containing, in addition to pantocid, anhydrous sodium carbonate and sodium chloride. One tablet is enough to neutralize 0.5-0.75 liters of water. For hand disinfection, 1-1.5% solutions are used.
Antiformin (Antiformimim). A mixture of equal amounts of 15% sodium hydroxide solution and 20% sodium hypochlorous solution (NaOCl). It is used for disinfection of infected material in laboratory practice and in dental practice for the treatment of ulcerative stomatitis (10-50% solutions).
Iodine tincture 5% (10%), FVIII. Alcohol 5 or 10% iodine solution. Applied externally. Inside is prescribed for the prevention of atherosclerosis, 1-10 drops.
Lugol's solution (Solutio Lugoli). Consists of 1 part iodine, 2 parts potassium iodide and 17 parts water. Used to lubricate mucous membranes.
Iodoform (Jodoformium), FVIII. Small shiny lamellar lemon-yellow crystals with a sharp characteristic persistent odor, almost insoluble in water, soluble in alcohol, ether, chloroform. It is applied externally in the form of ointments, powders and emulsions.
Oxidizers
Of the substances in this group, hydrogen peroxide, potassium hypochlorite and potassium permanganate are used as antiseptics. The mechanism of their antimicrobial action is based on oxidative capacity.
In tissues, hydrogen peroxide, due to the presence of the enzyme catalase, quickly decomposes to form molecular oxygen:
2H2O --- 2H2O = O2
The latter has a weak antimicrobial effect, so the use of hydrogen peroxide for the treatment of wounds is based mainly on the mechanical cleaning of wounds from pus with released oxygen bubbles. Hydrogen peroxide at topical application increases blood clotting, and therefore it is used to stop nosebleeds, introducing into the nasal cavity on tampons.
Potassium permanganate has a more significant antimicrobial effect than hydrogen peroxide. In low concentrations, it has an astringent effect, since the products formed during its restoration give complex compounds such as albuminates with proteins (see Astringents). Stronger concentrations of the drug have an irritating and cauterizing effect. Potassium hypochlorous acid (bertolet salt), sometimes used for gargling with sore throat, also has an antimicrobial effect.
Preparations
Hydrogen peroxide solution (Solutio Hydrogenii peroxydati diluta), FVIII. Clear colorless liquid containing 3% hydrogen peroxide. It is used for rinsing (a teaspoon or a tablespoon per glass of water) and washing wounds.
Perhydrol(Solutio Hydrogenii peroxydati concentrata), FVIII (B). Contains about 30% hydrogen peroxide. It is used for the manufacture of diluted solutions.
Hydroperite (Hydroperitum). A compound of hydrogen peroxide with urea containing about 33% hydrogen peroxide. When dissolved in water, it forms hydrogen peroxide. Available in tablets of 1.5 g containing 0.5 g of hydrogen peroxide. It is used for the manufacture of hydrogen peroxide solutions.
Potassium permanganate (Kalium hypermanganicum), FVIII. Dark purple crystals, soluble in water. As an antiseptic for rinsing and washing wounds, 0.01-0.5% solutions are used, for lubrication with burns, 2-5% solutions. In case of poisoning with alkaloids, the stomach is washed with 0.02-0.1% solutions of potassium permanganate.
Potassium hypochlorous acid (Kalium chloricum), FVIII. White crystalline powder, soluble in water. It is used for rinsing 1 teaspoon per glass of water.
Acids and alkalis
Some inorganic and organic acids are used as antiseptics. The antiseptic effect of inorganic acids depends on the degree of their dissociation. Lipoid-soluble inorganic and organic acids act more strongly than would be expected on the basis of their dissociation. Their action depends not only on the cation (H), but also on the anion. Acids and alkalis locally have an irritating and cauterizing effect on tissues, due to the fact that tissue proteins, reacting with both acids and alkalis, form albuminates. The effect depends on the degree of acid dissociation. With an increase in the degree of dissociation, the strength of the action of the acid on the tissue increases, and usually inorganic acids are stronger than organic acids. Some acids in low concentrations have an astringent effect.
When applied topically, salicylic acid has an antiseptic effect. Under the influence of weak concentrations salicylic acid(1-2%), epidermis proliferation occurs (keratoplastic effect), with an increase in concentration (10-20%), loosening and desquamation of the epidermis (keratolytic effect) is observed. Salicylic acid reduces the secretion of sweat glands. It is used externally for the treatment of various skin diseases, in the form of a corn plaster to remove calluses and in powders for excessive sweating.
Sulfuric, chromic, boric, acetic, trichloroacetic, benzoic, mandelic, undecylenic and some other acids are also used as antiseptics. Most of these acids are used externally, but some of them are used internally. Mandelic acid is administered orally to disinfect the urinary tract. Benzoic acid, often in the form of the sodium salt, is used as an expectorant. Many organic acids are used as flavoring agents.
Of the alkalis, caustic lime, ammonia, soda and borax are of greatest practical importance. Caustic lime is used in the form of milk of lime for external disinfection, as well as in the form of lime water as an astringent and antiseptic for burns and inflammation of the skin and internally for diarrhea. Ammonia is used to soak dirty linen and to treat the surgeon's hands before surgery (in the latter case, 0.25-0.5% solutions). The drug has a weak antiseptic and detergent effect. Soda and borax are used as weak antiseptic and mucus-cleansing agents.
Preparations
Salicylic acid (Acidum salicylicum), FVIII. White small crystals, sparingly soluble in water, soluble in alcohol. It is used in ointments (1-10%), powders (2-5%), alcohol solutions.
Benzoic acid (Acidum benzoicum), FVIII. Colorless, transparent crystals. Used in ointments. Benzoic acid is often used as an antimicrobial preservative in the manufacture of dosage forms.
Boric acid (Acidum boricum), FVIII. White fine crystalline powder. It is used in solutions (2%) for rinsing, washing the eyes, as well as in ointments and powders.
Undecin (Undecin).Ointment, which includes undecylenic acid and some other substances. Effective for fungal skin lesions (see Antifungal agents).
Glacial acetic acid (Acidum aceticum glaciale), FVIII. A colorless liquid that solidifies on cooling into a crystalline mass at a temperature of about +10°C. It is used to prepare solutions of acetic acid.
Diluted acetic acid (Acidum aceticum dilutum), FVIII. Contains about 30% acetic acid. It is used to prepare diluted solutions; A 5% solution of acetic acid has a strong bactericidal effect.
Trichloroacetic acid (Acidum trichloraceticum), FVIII. Colorless transparent crystals, used for cauterization in laryngological practice.
Piocidum (Pyocidum) (B).A liquid containing ether and anhydrous sulfuric acid. It is used in dental practice as a bactericidal agent.
Sodium borate (Natrium biboricum), FVIII. Colorless transparent crystals, soluble in water. It is used as an antiseptic for rinsing, douching and lubrication.
Sodium bicarbonate (Natrium bicarbonicum), FVIII. White crystalline powder, soluble in water. Externally used in 1-2% solutions for compresses and rinses, inside - in powder or tablets as an antacid for excessive acidity of gastric juice (see above).
Sodium carbonate (Natrium carbonicum). White loose powder, soluble in water. It is used for soaking dirty linen and for boiling surgical instruments.
Calcium oxide, burnt lime (Calcium oxydatum), FVIII. Amorphous pieces of white or grayish-white color, strongly heated when poured with water and turning into slaked lime (calcium oxide hydrate). Calcium oxide is slightly soluble in water. It is used for the manufacture of lime milk (10-20% suspension) and lime water.
Calcium hydroxide solution, lime water (Calcium hydrooxydatum solutum, Aqua calcis), OVIII. Saturated solution of calcium hydroxide in water (0.15-0.17%). It is used internally for diarrhea and externally in the form of calcareous liniment for burns and some other skin diseases.
Ammonia solution, ammonia (Ammonium causticum solutum, Liquor Ammonii caustici), FVIII. Colorless, transparent liquid with a pungent odor, containing about 10% ammonia. Used as such or after appropriate dilution (see Irritants).
Heavy metal compounds
Heavy metal compounds have both antimicrobial and characteristic local effects on body tissues (astringent, irritant, cauterizing effect). The action of salts of heavy metals depends on the ability of metal ions to form albuminates when interacting with proteins. The free acid is released as the second product of this reaction.
The nature of the local action of salts of heavy metals depends on the density of the resulting albuminate. Metals that give denser albuminates have a more pronounced astringent effect. The density of albuminate is due to the properties of the metal itself. On this basis, heavy metals are arranged in the following row: Al, Pb, Fe, Cu, Ag, Hg. The most dense albuminate is formed by aluminum salts, the most loose - by mercury salts.
An increase in the concentration of the solution is more often associated with the transition of an astringent action to a cauterizing one. The degree of dissociation of the compound also plays an important role. Other things being equal, a substance with a greater degree of dissociation has a more damaging effect on tissues than a compound that weakly dissociates. For example, mercury cyanide does little damage to tissues, and mercury dichloride at the same concentration has an irritating effect. With prolonged exposure to tissue, the damaging effect of the compound increases.
High concentrations of salts of heavy metals have a bactericidal effect. When using weak concentrations, a bacteriostatic effect is manifested. .
The antimicrobial effect of salts of heavy metals is mainly due to the fact that heavy metals block the sulfhydryl groups of the enzyme systems of the microbial cell, which causes inhibition of the growth and reproduction of microbes or their death.
While having much in common in terms of pharmacological properties, heavy metals nevertheless have individual differences. So, iron has an effect on the processes of hematopoiesis, silver is characterized by pronounced antiseptic properties, mercury and bismuth are used as specific chemotherapeutic agents in the treatment of syphilis.
After the absorption of large doses of salts of heavy metals, a toxic effect develops, characterized by inhibition of the function of the central nervous system, cardiac activity and expansion of capillaries.
This section will consider preparations of salts of heavy metals used as antiseptics and disinfectants.
Aluminum
In medical practice, aluminum as an astringent and antimicrobial agent is used in the form of salts of weak organic acids. A cauterizing effect can be obtained by using strong concentrations of aluminum salts.
Preparations
Burov's liquid (Liquor Burovi), FVIII. 8% solution of basic aluminum acetate salt, colorless transparent liquid. It is prescribed for rinsing, lotions and douching (the drug is diluted 5-10 times).
Alum (Alumen), FVIII. Double sulfate salt of potassium and aluminum. Colorless, transparent crystals, soluble in water. They are used in solutions (0.5-1%) for rinsing, lotions, douching as an astringent. As a cauterizing agent, they are used for trachoma (in the form of a pencil). Burnt alum (Alumen ustum) is used in powders as an astringent and in solutions for douching.
Lead
Like aluminum preparations, lead salts are used topically mainly as astringents.
When absorbed, lead has a toxic effect on the body. Therefore, people employed in industries where lead is used may experience occupational poisoning with this metal. The clinical picture of lead poisoning is diverse. One of early signs poisoning is a dark border on the gums. Its appearance is explained by the fact that lead is excreted by the mucous membranes of the digestive tract. In the mouth, lead reacts with hydrogen sulfide to form lead sulfide. Later develop anemia, lesions of peripheral nerves. There are also attacks of acute pain in abdominal cavity(lead colic from spasm of the intestinal musculature).
Preparations
Acetic lead (Plumbum aceticum), FVIII (B). Colorless crystals, soluble in water. It is used in aqueous solutions (0.25-0.5%) as an astringent.
Lead water, lead lotion (Aqua Plumbi), FVIII. 2% aqueous solution of basic lead acetate. It is used for lotions and compresses.
Bismuth
The local action of bismuth salts differs from the local action of salts of other heavy metals in that they do not have an irritating and cauterizing effect. Bismuth has a bacteriostatic effect on microbes, which is explained by the binding of thiol groups (-SH) of enzyme systems of microbial cells by bismuth ions. When administered orally, bismuth preparations reduce peristalsis, since bismuth binds hydrogen sulfide, which is a natural causative agent of peristalsis. As a result, an antidiarrheal effect occurs. The deposition of insoluble bismuth sulfide on the intestinal wall also contributes to the weakening of peristalsis. Bismuth also has an antimicrobial effect in the intestine. In this regard, bismuth preparations are prescribed orally for inflammatory processes in the intestine. Bismuth is not absorbed from the intestine. Its resorptive effect is manifested by parenteral administration (see Chemotherapeutic agents).
Preparations
Basic bismuth nitrate (Bismutum nitricum basicum, Bismutum subnitricum), FVIII. White amorphous powder. It is prescribed orally at 0.25-0.5 g 3-4 times a day or in powders and ointments.
Xeroform (Xeroformium), FVIII. The basic bismuth tribromophenolate is a fine yellow powder containing 50% bismuth oxide. It is used in ointments, powders. Included in Vishnevsky's ointment (tar 3 parts, xeroform 3 parts, castor oil 100 parts), used to treat wounds.
Dermatol (Dermatolum), FVIII. Basic bismuth salt of gallic acid. Powder of lemon-yellow color, contains over 50% bismuth oxide. It is prescribed in powders, ointments (10%), suppositories (0.2 g each).
Copper and zinc
Copper and zinc salts are similar in their pharmacological properties. When applied topically, depending on the strength of the solution, they have an astringent, irritating and cauterizing effect. Copper and zinc also have antiseptic properties. Zinc and copper sulfates are widely used for conjunctivitis (inflammation of the mucous membrane of the eyes) as an antiseptic and astringent. When ingested, they cause vomiting (see Vomiting).
Preparations
Copper sulphate (Cuprum sulfuricum), FVIII. Blue crystals, soluble in water. A 0.25% solution is used as an astringent. Stronger solutions have a cauterizing effect. In trachoma, Cuprum sulfuricum alumina turn (an alloy of copper sulphate, saltpeter, alum and camphor) is used to cauterize the conjunctiva. As emetic appoint repeatedly 0.1 g in 1% solution.
The highest single dose inside: 0.5 g.
Copper citrate (Cuprum citricum), FVIII. Light green powder. It is used for trachoma in eye ointments (1-5%).
Zinc sulfate (Zincum sulfuricum), FVIII. Colorless crystals, soluble in water. As an astringent in eye practice, a 0.25% solution is used. Sometimes it is used as an emetic inside of 0.1-0.3 g in a 1% solution.
The highest single dose (orally) as an emetic: 1 g.
Zinc oxide (Zincum oxydatum), FVIII. White powder, insoluble in water. It is used in ointments, pastes and powders. Included in the paste Lassara.
Mercury
The mechanism of the antimicrobial action of inorganic and organic mercury compounds is based on their blocking of sulfhydryl groups that are part of the enzyme systems of the microbial cell, as well as on the disruption of the biochemical function of thiamine and some amino acids (histidine, glutamic acid, methionine). The inhibitory effect of mercury on microbes is eliminated by sulfhydryl compounds and thiamine. Under the influence of low concentrations, a bacteriostatic effect develops. With an increase in the concentration of the solution and the duration of its contact with the microbe, a bactericidal effect occurs. Among mercury compounds, sublimate, or mercury dichloride, is the most powerful antiseptic, which is associated with a high degree of dissociation of the compound. The strength of the antimicrobial action of sublimate decreases in the presence of protein.
Sublimate is not used to disinfect metal instruments, as it causes corrosion of metals. Sublimate has an irritating effect on tissues, especially with repeated use. Oxycyanic mercury with a low degree of dissociation does not irritate tissues and has a bacteriostatic effect.
Mercury compounds are strong poisons for animals and humans. In acute poisoning, circulatory disorders and paralysis of the nervous system are observed. In subacute poisoning, there is a lesion internal organs: kidneys, intestines, etc. Possible tissue damage at the injection site. In chronic poisoning with mercury compounds (mercurialism), a complex pattern of damage to various organs and tissues develops: ulcerative stomatitis, colitis, headaches, irritability, muscle tremors, mental disorders.
Preparations
Mercury dichloride (Hydrargyrum bichloratum), FVIII (A). White powder, soluble in water. For disinfection of care items, linen, solutions of 1:1000 or 1:500 are used. Produced in tablets, tinted with eosin (0.5 and 1 g of sublimate) for the preparation of solutions.
Higher doses: 0.02 g (0.08 g).
Mercury oxycyanide (Hydrargyrum oxycyanatum), FVIII (A). White powder, soluble in water. It is used in eye practice for washing in solutions of 1:5000 and 1:10000.
Amidochloric mercury, white sedimentary mercury (Hydrargyrum amidatochloratum, Hydrargyrum praecipitatum album), FVIII (B). White amorphous powder. It is used in ointments (5-10%) for skin diseases and as cosmetic product(to remove freckles).
Mercury oxide yellow (Hydrargyrum oxydatum flavum), FVIII (B). Yellow powder. It is used in ointments for eye diseases (2%) and for skin diseases.
Diocide (Diocidum) (A).A mixture of cetylpyridinium bromide and ethanolmercury chloride. Cetylpyridinium bromide is a cationic soap (see below). Diocide is proposed for the treatment of hands before surgery. It is a strong antiseptic, provides asepsis for a period of at least 2 hours. Apply solutions 1:3000-1:5000.
Silver
Silver compounds are characterized by significantly pronounced antimicrobial properties, especially in relation to the coccal group of bacteria. As an antiseptic, silver nitrate is most widely used. In low concentrations, it has an astringent and anti-inflammatory effect. Strong solutions (1% and above) of silver nitrate act on cauterized tissues.
Interacting with proteins, silver nitrate forms a dense albuminate, which gradually acquires a black color, which is associated with the reduction of silver. Silver nitrate is used in surgery for the treatment of wounds (as a cauterizing agent with excessive formation of granulation tissue), in ophthalmic practice for the prevention of neonatal blennorrhea (1 drop of a 2% solution is instilled into each eye). Sometimes it is prescribed orally for peptic ulcer disease. Colloidal preparations of silver - collargol and protargol - do not form albuminates. These drugs are used as antiseptic and anti-inflammatory drugs.
Preparations
Silver nitrate, lapis (Argentum nitricum), (PVIII (A). Colorless transparent crystalline plates, soluble in water. It is used in aqueous solutions (1-10%) or in the form of sticks (Stilus Argenti nitrici) for cauterization. Inside, it is used as an astringent, 0.01 g 2-3 times a day in solutions (0 05%).
The highest single dose inside: 0 03 g (0.1 g).
Protargol (Protargolum), FVIII. Brownish-yellow powder, soluble in water, containing about 8% silver. It is used in solution (0.5-5%) for diseases of the mucous membranes of the eyes, upper respiratory and urinary tract.
Collargolum, FVIII (B). colloidal silver. The drug contains 70% silver. For washing purulent wounds, 0.2-1% solutions are used, in eye drops - 2-5%, in a vein - 2% solution of 2-10 ml.
Highest doses in a vein: 0.25 g (0.5 g).
Alcohols, aldehydes
Pharmacological properties of ethyl alcohol are discussed in the chapter "Narcotic drugs". Ethyl alcohol is widely used as an antiseptic.
Formaldehyde- a gaseous substance. For medical purposes, a 40% aqueous solution of formaldehyde, called formalin, is used. Formalin has a pronounced antimicrobial effect, inhibiting both vegetative forms of bacteria and spores. It causes protein denaturation, which is the reason for its local irritant effect. Formalin reduces the secretion of sweat glands. It is mainly used for external disinfection both in solutions and by the paraformalin method.
Urotropin- hexamethylenetetramine - by itself does not have an antimicrobial effect, but in an acidic environment it decomposes into ammonia and formaldehyde. The formation of the latter explains the antiseptic effect of urotropin. The breakdown of urotropin in the body occurs in the kidneys, as well as in places where there is an inflammatory process, the development of which, as you know, is accompanied by a shift in the reaction of the environment to the acid side. Urotropin is prescribed orally and intravenously for infectious diseases, especially the urinary tract.
Preparations
Formalin(Formalinum, Formaldehydum solutum), FVIII. 40% solution of formaldehyde in water, a clear liquid with a peculiar pungent odor that irritates mucous membranes. It is used in solutions as a disinfectant and antiseptic (0.5-1%), for fixing anatomical preparations (10-15%) and with excessive sweating of the hands and feet (0.5-1%), as well as for steam-formalin disinfection. For the latter purposes, in addition, paraform is used - a solid polymer of formaldehyde.
Lysoform (Lysoformium), FVIII. Soapy formaldehyde solution. For disinfection of hands and premises, 2-3% solutions are used, for douching 1-4% solutions.
Urotropin (Urotropinum), FVIII. Colorless crystals. Inside designate 0.5-1 g, intravenously - 5-10 ml of a 40% solution.
Phenols and products of dry distillation of organic materials
Phenol.The antimicrobial properties of phenol, or carbolic acid, like other antiseptics, depend on a number of conditions. The solvent plays an important role. Aqueous solutions have the highest activity, alcohol and especially oil solutions are inactive. As the temperature rises, the antimicrobial properties increase. In low concentrations (1:400-1:800) phenol has a bacteriostatic effect, 1-5% phenol solutions cause the death of microbes. Not all types of microbes are equally sensitive to phenol. Spores are insensitive to phenol. In the presence of protein, the antimicrobial effect of phenol changes little, which is an advantage of phenol over other antimicrobial agents.
Locally on the tissue, phenol has an irritating effect; with increasing concentration, the development of necrosis is possible. Initially, there is acute pain, followed by anesthesia.
Phenol is easily absorbed through mucous membranes and wound surfaces. Absorption is also possible through intact skin. Phenol after absorption into large quantities causes acute poisoning. Symptoms of poisoning when taking phenol inside: nausea, vomiting, necrosis in the mouth and stomach, acute pain, loss of consciousness, a sharp drop in temperature, blood pressure and respiration. Seizures may occur. The immediate cause of death is respiratory paralysis.
In case of poisoning, it is necessary to wash the stomach, give inside lime sugar (Calcaria saccharata). With depression of the central nervous system, stimulants are prescribed.
Phenol is used to disinfect hands, rooms, tools, and in low concentrations (0.25-0.5%) - as a preservative.
The phenyl ester of salicylic acid is saponified in the intestine to form phenol and salicylic acid. The drug is used orally as an antiseptic for the intestines, biliary and urinary tract.
Similar to salol, the drug benzonaphthol (naphthyl ester of benzoic acid) is saponified in the intestine to form betanaphthol, which has an antiseptic effect on the contents of the intestine.
Methylphenolsor cresols(three isomers) are similar in properties and action to phenol. They are distinguished from it by low solubility and poor absorption, but cresols are superior to phenol in terms of antimicrobial action. The solubility of cresols increases in an alkaline environment.
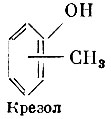
Cresols are used in soap solutions for the disinfection of linen, rooms, furniture, and also for the preservation of solutions for subcutaneous administration.
Or meta-dioxiphenol, less toxic than phenol, and somewhat inferior to it in terms of antimicrobial action.
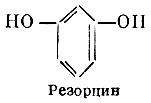
In low concentrations, resorcinol causes a keratoplastic effect, from stronger concentrations, a keratolytic effect is observed. Resorcinol is used externally in the form of ointments and solutions for skin diseases.
It's pretty strong antibacterial action, however, in practice it is mainly used as an anthelmintic agent (see below).
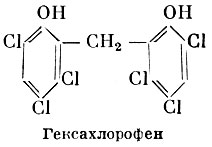
Chlorine-substituted and some other phenol derivatives have a strong antimicrobial effect, often significantly superior in activity to carbolic acid. Among phenol derivatives, one should mention hexachlorophene (2,2"-dioxy-3, 5, 6, 3", 5", 6"-hexachlorodiphenylmethane), which has a high bactericidal activity and does not irritate the skin. Hexachlorophene is used to make disinfectant soap used for washing hands.
Bearberry leaf (Arctostaphylos uva ursi) contains the glucoside arbutin, which breaks down in the body to form a diatomic phenol - hydroquinone (paradioxibenzene). Excreted by the kidneys, hydroquinone has an antiseptic effect on the urinary tract and causes a diuretic effect.
tarof various origin - products of dry distillation of wood - have a complex composition. Their antiseptic effect depends on the content of phenols in them (phenol, cresols, guaiacol, creosol, etc.).
In addition to a purely antiseptic effect, tars have a local irritant and keratoplastic effect, as well as an insecticidal effect.
Of the other products of dry distillation, ichthyol and albichtol are of practical importance (see Preparations).
Preparations
Pure phenol, crystalline carbolic acid (Phenolum purum, Acidum carbolicum crystallisatum), FVII (B). Colorless crystals, gradually turning pink in the air. For disinfection, 3-5% solutions are used, for the preservation of medicinal substances and forms - 0.1-0.3% solutions.
Pure liquid phenol, liquid carbolic acid (Phenolum purum liquefactum, Acidum carbolicum liquefactum), FVIII (B). Colorless or pinkish oily liquid. 100 parts of phenol contains 10 parts of water.
Tricresol (Tricresolum), FVIII (B). A mixture of ortho-, meta- and para-cresols. Colorless or light yellow liquid with a characteristic odor. It is used for disinfection, like phenol, as well as for the preservation of injection solutions.
Lysol medical (Lysolum medicinale), FVIII. Transparent oily liquid of red-brown color, which is a solution of cresol in potassium soap. For disinfection prepare 3-10% solutions. For disinfection of hands and for douching, 0.5-1% solutions are used.
Resorcinol (Resorcinum), FVIII Colorless crystalline powder, soluble in water and alcohol. For skin diseases, 2-5% aqueous and alcoholic solutions, 5-10% ointments are used. Sometimes resorcinol is prescribed orally as an antiseptic for the gastrointestinal tract.
Hexachlorophene soap. Toilet soap containing hexachlorophene. Used for hand washing for disinfection.
Bearberry leaf (Folium Uvae ursi), FVIII. Small, leathery, dense, brittle leaves. Used as a decoction (1:10 or 1:20) for inflammatory diseases of the urinary tract.
Salolum, FVIII.White crystalline powder, almost insoluble in water. Assign inside of 0.3-0.5 g 2-3 times a day for nonspecific infectious diseases of the intestine.
Benzonaphthol (Benzonaphtholum), FVIII. White fine-crystalline powder, odorless and tasteless, insoluble in water. It is applied orally at 0.3-0.5 g 3 times a day.
Ichthyol(Ichthyolum, Ammonium sulfoichthyolicum), FVIII. It is obtained as a result of processing shale tar - a product of dry distillation of special types of slates. Contains ammonium salts of shale oil sulfonic acids. A brown syrupy liquid that has anti-inflammatory, local anesthetic and antiseptic effects. It is used in ointments (5-30%), suppositories, balls, on tampons mixed with glycerin.
Albichtol (Albichtolum), FVIII. A transparent mixture of thiophene homologues with an admixture of hydrocarbons. Yellowish liquid. It is used in ointments (2-15%), candles and balls. In terms of pharmacological properties, it is similar to ichthyol. It is used in combination with green soap in the form of a paste to combat lice, bedbugs and cockroaches.
Dyes
A feature of the antimicrobial action of dyes is the well-known selectivity of their action on certain groups of microbes, which consists in the fact that some microorganisms are especially sensitive to the action of certain paints. The group of dyes includes brilliant green, rivanol, tripaflavin and methylene blue.
According to the chemical structure, it belongs to the derivatives of rosaniline, or triphenylmethane (oxalatee). Brilliant green has a high antimicrobial activity against Staphylococcus aureus, the causative agent of diphtheria and other gram-positive bacteria. The presence of organic compounds in the environment dramatically reduces the antimicrobial effect of the drug. It is used externally as an antiseptic for purulent skin lesions.
An acridine derivative (2-ethoxy-6,9-diaminoacridine lactate), effective as an antiseptic for infections caused by coccal flora, especially streptococci. It is used in aqueous solutions for prophylactic and therapeutic purposes for washing cavities, in the form of tampons, lotions, eye drops, as well as for skin diseases in ointments and lotions. Rivanol in the applied concentrations has a predominantly bacteriostatic effect. When applied topically, tissues are not irritating. The overall toxicity of rivanol is low.
Tripaflavin, or flavacridine (a mixture of 3,6-diaminoacridine hydrochloride and its 10-chloromethylate), has a great antimicrobial effect, has a depressing effect on the causative agent of diphtheria and coccal flora (streptococci, staphylococci, meningococci, gonococci). Tripaflavin is used, as well as a chemotherapeutic agent (see Medications for the treatment of protozoal infections), for animal piroplasmosis (intravenously). Blood serum does not reduce the antimicrobial activity of tripaflavin. In moderate concentrations, tripaflavin does not irritate tissues. It is applied topically in the form of lotions and washes for the treatment of infected wounds, phlegmon, abscesses. Previously, tripaflavin was used to treat sepsis, meningitis, endocarditis and other diseases (the drug was administered intravenously, with caution).
Tripaflavin is excreted by the kidneys, turning urine green.
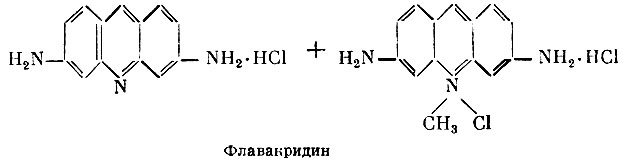
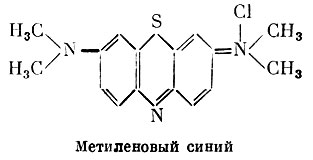
Tetramethylthionine chloride is inferior to other drugs in this group in terms of antiseptic properties. It is used as an antiseptic, externally for burns, purulent skin diseases. Ingestion is indicated for inflammatory diseases of the urinary tract. Methylene blue is also used as an antidote for hydrocyanic acid poisoning. The therapeutic effect is based on the ability of methylene blue to convert hemoglobin to methemoglobin. Methemoglobin, in turn, enters into a strong connection with cyanides and thereby eliminates their effect on body tissues.
Preparations
Brilliant green (Viride nitens), FVIII. Golden-green powder, soluble in water and alcohol. It is used in water and alcohol solutions (1-2%) for lubrication.
Rivanol (Rivanolum), FVIII (B). Yellow fine crystalline powder, soluble in water. For the treatment of wounds, 0.05-0.2% aqueous solutions are used, for washing cavities - 0.05-0.1% solutions. Ointments and pastes may contain up to 10% rivanol.
Tripaflavin (Trypaflavinum), FVIII (B). Orange-red crystalline powder, soluble in water and alcohol. Locally applied 0.1% solution of tripaflavin in water or isotonic sodium chloride solution.
Methylene blue (Menthylenum coeruleum), FVIII. Dark green crystalline powder. Externally applied 1-3% alcohol solutions. Inside is assigned to 0.1 g 3-4 times a day.
As an antidote, Methylene blue is administered intravenously in 50-100 ml of a 1% solution prepared in a 25% glucose solution (this solution is called chromosmon).
Nitrofuran derivatives
Nitrofuran derivatives are new class compounds with bacteriostatic activity.
The antimicrobial effect of nitrofuran derivatives is due to the presence of an aromatic nitro group in the molecule. Distinctive feature derivatives of nitrofuran is a wide antibacterial spectrum of action (see Antibiotics). They have an inhibitory effect on gram-positive and gram-negative bacteria, some large viruses and protozoa. During the last 10-15 years, a large number of compounds of this series have been synthesized.
Furacilin- 5-nitro-2-furfurylidene-semicarbazone, has a wide antibacterial spectrum, has a depressing effect on both gram-positive and gram-negative bacteria. Among them are Escherichia coli, staphylococci, streptococci, paratyphoid bacilli, the causative agent of gas gangrene. Furacilin has an inhibitory effect on penicillin and sulfanilamide-resistant races of microbes (see Penicillin and Sulfanilamides). The resistance of microbes to furacilin develops slowly. The mechanism of the antimicrobial action of furacilin is based on the inhibition of dehydrogenases - enzymes involved in redox processes.
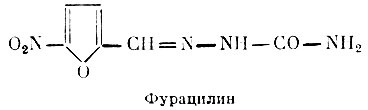
Locally, furatsilin in the applied concentrations does not have an irritating effect on the tissue. On the contrary, by enhancing the formation of granulation tissue and the process of epithelialization, it promotes wound healing. Furacilin is widely used in surgical, gynecological and urological practice for the prevention of purulent infection, as well as for the treatment of various purulent processes.
Cavities are washed with aqueous solutions of furacilin, re-irrigate wound surfaces, purulent and surgical wounds, soak dressings, tampons. With dysentery, the drug is prescribed orally.
The positive properties of furacilin include its resistance to high temperatures.
Another drug of the nitrofuran series is ntrofurantoin - N-(5-nitro-2-furfurylidene)-aminohydantoin.
Nitrofurantoin has a broad antibacterial spectrum, but does not affect fungi and viruses. When taken orally, it is well absorbed and rapidly excreted in the urine in an amount of 50% of the dose taken. It is almost not excreted with feces. Nitrofurantoin can cause nausea and vomiting. It is used for the oral treatment of urinary tract infections.
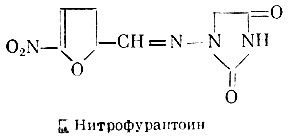
The next drug in this series is furazolidone N-(5-nitro-2-furfurylidene)-3-amino-2-oxazolidone. It proved to be useful for the treatment of trichomonas colpitis. Furazolidone is applied by insufflation into the vagina of powdered sugar containing 0.1% of the drug.
Preparations
Furacilin (Furacilinum) (B). Yellow crystalline powder. It is applied externally in solutions of 1:5000. With purulent otitis media, an alcohol solution of 1:1500 is instilled into the external auditory canal. In eye practice, an ointment with a furacilin content of 1:500 is used. Sometimes prescribed inside of 0.1 g 4 times a day (with dysentery).
Of the derivatives of oxyquinoline, chinosol (8-hydroxyquinoline sulfate) and yatren are used as antiseptics (see Chemotherapeutic drugs). Quinosol is also used as contraceptive. Locally on the tissue, chinosol does not have an irritating effect.
The antimicrobial effect of 8-hydroxyquinoline is explained by its ability to form complex compounds with metals that are of great importance for biochemical reactions occurring in the cell.
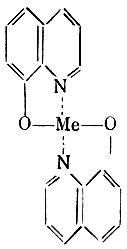
The entry of metal into such compounds (pincer formation) makes it biologically inactive.
Preparations
Chinosol (Chinosolum), FVIII. Fine-crystalline powder of lemon-yellow color. For washing wounds, ulcers and douching, solutions of 1:1000-1:2000 are prepared. As a contraceptive, chinosol is used in balls (0.2 g each).
Surfactants
Many surfactants, or detergents, have detergent, foaming and emulsifying properties, and therefore are widely used in industry as detergents and emulsifiers. Along with this, detergents dissociating in solutions have an antimicrobial effect.
There are cationic, anionic and non-ionic detergents. In the first case, the surface activity is determined by the properties of the cation, in the second, by the properties of the anion. Cationic detergents are widely used in medical practice as antiseptics. According to their chemical structure, they belong to salts of Quaternary ammonium bases. The antimicrobial action of these compounds is based, on the one hand, on their ability to lower surface tension, on the other hand, it is possible that a decrease in the activity of a number of enzyme systems of a microbial cell also plays a role. The presence of protein in the medium sharply reduces the antiseptic properties of the compound. Cationic detergents are relatively low toxic.
In the Soviet Union, diocide is used as a bactericidal agent for washing the hands of a surgeon. It contains one of the representatives of this group of substances - cetylpyridinium bromide and a mercury compound (see Mercury).
Chemotherapeutic agents
I
drugs that selectively suppress the development and reproduction of pathogens of infectious diseases and invasions in the human body or inhibit the proliferation of tumor cells or irreversibly damage these cells.
As H. with. use substances of natural origin: Antibiotics and some alkaloids, such as quinine and emetine, as well as synthetic substances from various classes of chemical compounds: sulfonamides (see.Sulfanilamide preparations), nitrofuran derivatives (see Nitrofurans ), 8-hydroxyquinoline (seeOxyquinoline derivatives), nitroimidazole, aminoquinoline, etc.
In connection with the fundamental differences between the infectious and tumor processes, Ch.Anticancer drugs).
The mechanism of action of various H. with. unequal. X. s. can affect different elements of a microorganism cell: the cell wall, the cytoplasmic membrane, the ribosomal apparatus that provides intracellular protein synthesis, nucleic acids and some enzymes that catalyze the formation of substances necessary for the life of cells. So, some antibiotics (penicillins, cephalosporins, cycloserine) and synthetic antifungal drugs (miconazole, ketoconazole, etc.) disrupt the synthesis of the cell wall of microorganisms. The molecular organization and functions of cytoplasmic membranes are violated by polymyxins, some antifungal antibiotics of a polyene structure: amphotericin B, nystatin, levorin, etc. Protein synthesis at the ribosome level is inhibited by antibiotics of the aminoglycoside group, chloramphenicol, tetracyclines. The synthesis and functions of nucleic acids in microorganisms are disrupted by rifamycins, griseofulvin, ethambutol, and chingamine. Some antiviral agents, such as idoxuridine and vidarabine, can affect DNA metabolism. Row H. s. acts on the principle of antimetabolites. Thus, sulfanilamide preparations are competitive antagonists of para-aminobenzoic acid and replace it in the synthesis of folic acid, which is involved in the synthesis of purines and pyrimidines. The mechanism of action of chloridine and trimethoprim is associated with the inhibition of dihydrofolate reductase, which catalyzes the conversion of folic acid to tetrahydrofolic acid. Used as H. with. bismuth preparations, such as biyoquinol, bismoverol, antimony compounds, such as solusurmin, etc., block the sulfhydryl groups of various enzymes of microorganisms.
When creating new H. with. proceed from the following requirements for them: high selectivity of the antimicrobial effect in non-toxic doses for humans (high chemotherapeutic index); slow development of drug resistance in microorganisms (Drug resistance of microorganisms); maintaining high activity in different environments of the body: optimal pharmacokinetic properties (absorption, distribution, excretion) that ensure the accumulation of Ch. in the foci of localization of pathogens in quantities sufficient to suppress the vital activity of microorganisms, etc. Obtaining Ch. In this regard, most of the existing H. s. has certain disadvantages that must be taken into account in the process of using drugs.
In medical practice H. with. widely used for etiotropic therapy of patients with infectious diseases (see. Chemotherapy ), as well as for the prevention of infections (see.Chemoprophylaxis) and sanitation of persons who are carriers of certain pathogens (chemosanation).
In the process of applying H. s. can provide side effect. All caused H. with. side effects can be divided into three groups: 1) allergic reactions; 2) reactions caused by the direct toxic effect of Ch. 3) reactions associated with the specific (antimicrobial) action of Ch.
Like most others medicines, X. s. are chemical compounds alien to the human body and therefore can act as antigens. By their nature, caused by H. s. allergic reactions do not differ from similar reactions caused by any other drugs. The symptoms of these reactions are characterized by polymorphism from itching, urticaria and other drug-induced dermatitis to the most severe anaphylactic reactions such as angioedema and anaphylactic shock. Similar complications develop in persons sensitized to a particular drug. In this regard, in order to prevent them before the appointment of H. s. it is advisable to establish whether there was a history of any allergic reactions to the prescribed drug or drugs similar in structure to it, since cross-allergy usually develops to substances of a similar chemical structure. For example, to all drugs of the penicillin group, sulfonamides, etc.
In addition to specific (antimicrobial) activity, X. s. have a certain organotropism, which is the reason for the development of side effects associated with their direct toxic effect. Such effects are typical for individual drugs (for example, aminoglycoside ototoxicity, polymyxin nephrotoxicity, etc.). The degree of their severity and frequency of occurrence largely depend on the dose, route of administration and duration of use of the drugs.
Side effects of this group include local reactions arising from the direct irritant effect of drugs in the area of their administration, for example, aseptic abscesses and necrosis when intramuscular injection, phlebitis - with intravenous administration, dyspeptic disorders - when taking drugs inside. The same group of complications includes toxic lesions of individual organs or systems, for example, neurotoxic, hepatotoxic, nephrotoxic reactions, etc.
Neurotoxic reactions may occur mental disorders(acriquine, isoniazid, cycloserine), lesions of the VIII pair of cranial nerves (aminoglycosides, quinine), optic nerve (quinine, emetine, ethambutol), polyneuritis (isoniazid, cycloserine, polymyxin, emetine), etc. Nephrotoxic effect is typical for aminoglycosides, polymyxins, sulfonamides, amphotericin B, griseofulvin and some other drugs. Isoniazid, sulfonamides, rifamycins, tetracyclines, amphotericin B, erythromycin have hepatotoxic properties. Sulfonamides, levomycetin, amphotericin B, chloridine can have a negative effect on hematopoiesis. In persons with congenital deficiency of glucose-6-phosphate dehydrogenase in erythrocytes, some H. s. (eg, quinine, primaquine, sulfonamides) may cause hemolytic anemia.
TO side effects associated with the antimicrobial action of Ch. . Complications of this group occur only when using H. s. and do not develop under the influence of other drugs that do not have antimicrobial activity.
Dysbacteriosis develops as a result of a violation under the influence of Ch. normal biological balance of microflora in the body. for example, when suppressed by antibiotics a wide range the action of saprophytic bacterial flora creates conditions for the excessive development of yeast-like fungi and the occurrence of candidiasis. Complications of this kind do not develop when using H. s. with a limited spectrum of antimicrobial activity (for example, synthetic anti-tuberculosis drugs - isoniazid, etc., antimalarial drugs, griseofulvin and a number of other drugs).
The reaction of bacteriolysis, or endotoxic reaction (Yarish-Herxheimer reaction), occurs as a result of the rapid death of pathogens and the release of a large amount of endotoxins from them. It is manifested by chills, fever, profuse sweating, and some other symptoms resembling those of endotoxic shock. This complication can occur with a number of infections (typhoid fever, syphilis, brucellosis, etc.) at the beginning of treatment with active Ch. in high doses.
The cause of vitamin deficiency when using H. s. most often, they suppress the vital activity of the intestinal microflora, which synthesizes a number of vitamins - riboflavin, pyridoxine, etc. However, some H. s. can cause hypovitaminosis and due to other mechanisms. Thus, isoniazid disrupts the formation of pyridoxal phosphate and thereby contributes to the development of signs of pyridoxine deficiency.
With vigorous chemotherapy with highly active H. s. such a rapid suppression of the pathogen is possible that, at the same time, sufficient tension of cellular or humoral immunity does not have time to develop. This is one of the reasons for the occurrence of relapses in certain infections - brucellosis, typhoid fever, etc. In addition, some Ch.
II Chemotherapeutic agents
drugs that suppress the vital activity of microorganisms or tumor cells (antibiotics, sulfonamides, antitumor agents, etc.).





